Portfolio
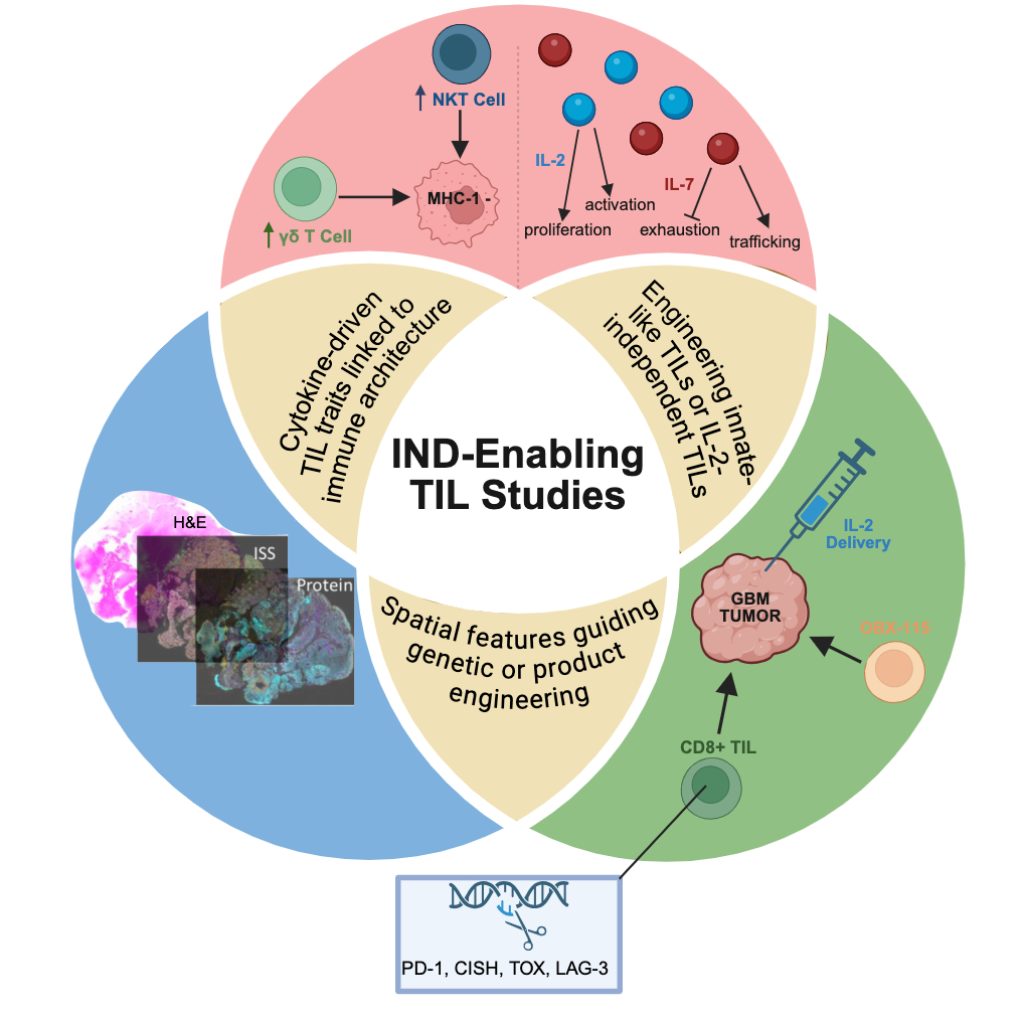

Our Research Initiatives
Our focus is tumor immunobiology, leveraging biologically relevant tumor-immune interactions to bring new therapies to patients.
Why our work matters
Glioblastoma is an aggressive brain tumor with an average survival of only 18–21 months, even with the best standard treatments. Many patients run out of options quickly. Our lab is working to change that by:
- Designing trials for newly diagnosed patients (like ETAPA and GIANT) before the disease worsens
- Bringing the latest science directly into patient care
- Building a future where better, personalized treatments are available for all brain tumor patients
Our Partnerships
The Khasraw lab collaborates extensively with researchers across Duke and beyond – including internationally – to drive innovation and expand the reach of our work. Many of our clinical trials are multicenter, enabling broader patient recruitment and more robust results. We’re deeply grateful to all of our partners for their shared commitment to improving patient outcomes.